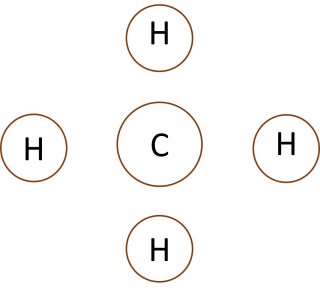
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.46 Activity 2. Crossing and dotting Students should: 1.46 understand how to use dot-and-cross diagrams to represent covalent bonds in molecules. Dot and cross diagrams are a useful way of representing how atoms share electro...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.44 - 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the ...
Covalent bonds form when electrons are shared between nuclei Atoms which can form two or more covalent bonds can bond with other similar atoms or themselves and form covalent lattices. Carbon in the form of diamond is a good example, as is silicon dioxide in the form of quartz. A valuable lattice Activity. Diamond - clos...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the video t...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.