1.23 -1.24 Grouping together
1.23 Family resemblances
Students should:
1.23 understand why elements in the same group of the Periodic Table have similar chemical properties
1.24 understand why the noble gases (Group 0) do not readily react
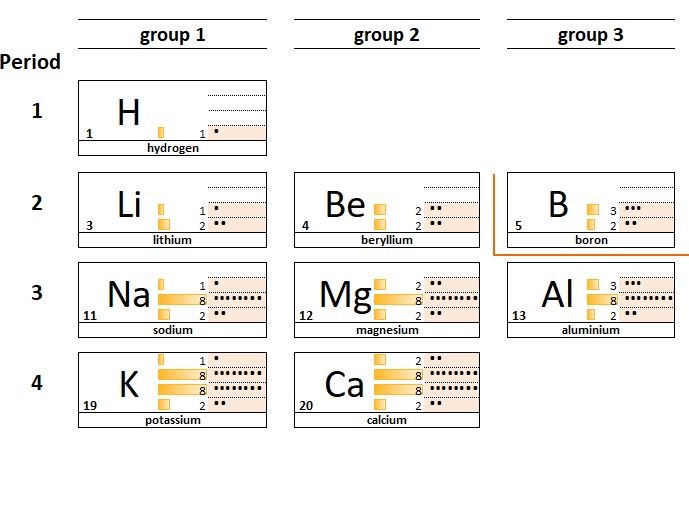
Looking at the electron arrangements of the atoms in groups 1, 2 and 3 you can see that the atoms of group 1 elements have 1 electron in their outermost shell, group 2 elements all have 2 electrons and group 3 elements 3. This carries on in groups 4,5,6,7.
This similarity of electron configuration within a given group of elements explains why the elements in a group behave in similar ways; they have similar physical and chemical properties.
For example: lithium Li , sodium Na, and potassium K :
- all react violently with water.
- are all soft low density metals
- combine with chlorine atoms in a 1:1 ratio
- form oxides which dissolve to give an alkaline solution
1.24. Activity 4. A Noble family
The chemical behaviour of a element is partly determined by the number of electrons in the outer shell.
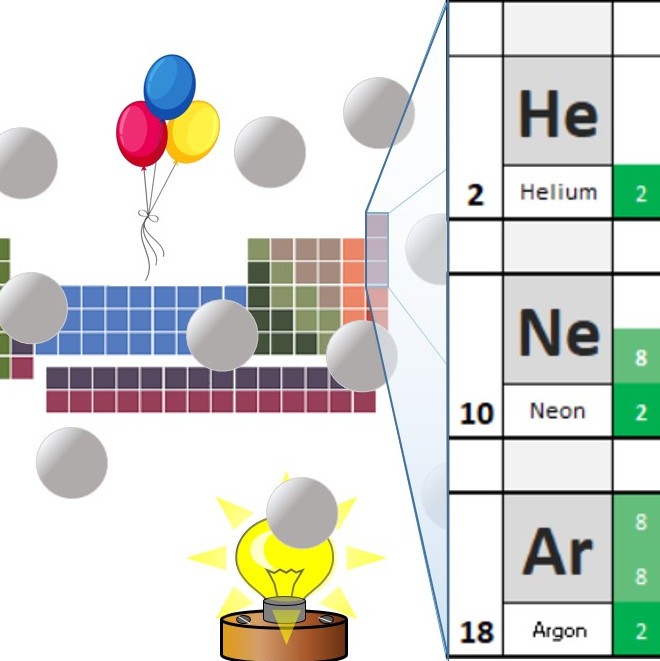
In the noble gases or group 0 the outermost shell of the atoms contains 8 electrons (2 in case of Helium) which is a stable state configuration.
Hence, the noble gases are chemically unreactive or inert.
noble, inert, 8 or 0?
In the shortened periodic table, the group number is the same as the number of outer shell electrons in the atoms of that group. This is not true for the noble gases. Helium only has 2 electrons in its outer shell, while the others all have 8 . Because of this, the group number was changed to 0 .
Originally the group was known as the inert gases as it was thought that they would not react with anything. However in 1962 a chemist called Bartlett made a compound from Xenon. The preferred name for the group is now the noble gases.
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.