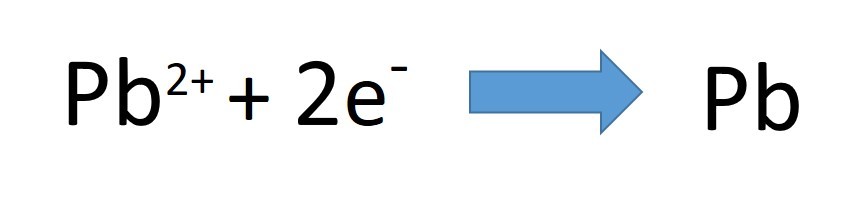1.55 - 1.60 Electrolysis
1.55 Using electrolysis
Electrolysis happens when an electric current is passed through an ionic compound which has been melted or dissolved.
Electrolysis has a wide range of uses including : electroplating, extraction of aluminium, production of chlorine and the purification of copper . This post helps you to understand and explain the various changes that can happen in electrolysis.
Assumed background knowledge
https://www.goldhilleducation.co.uk/index.php/private-tuition/formulae-of-compounds
How metals conduct an electric current:
The delocalised electrons in the metallic lattice are free to move through the material when a voltage is applied.
1.55 - 1.57 Activity 1. Know your anions
Students should:
- 1.55C understand why covalent compounds do not conduct electricity
- 1.56C understand why ionic compounds conduct electricity only when molten or in aqueous solution
- 1.57C know that anion and cation are terms used to refer to negative and positive ions respectively
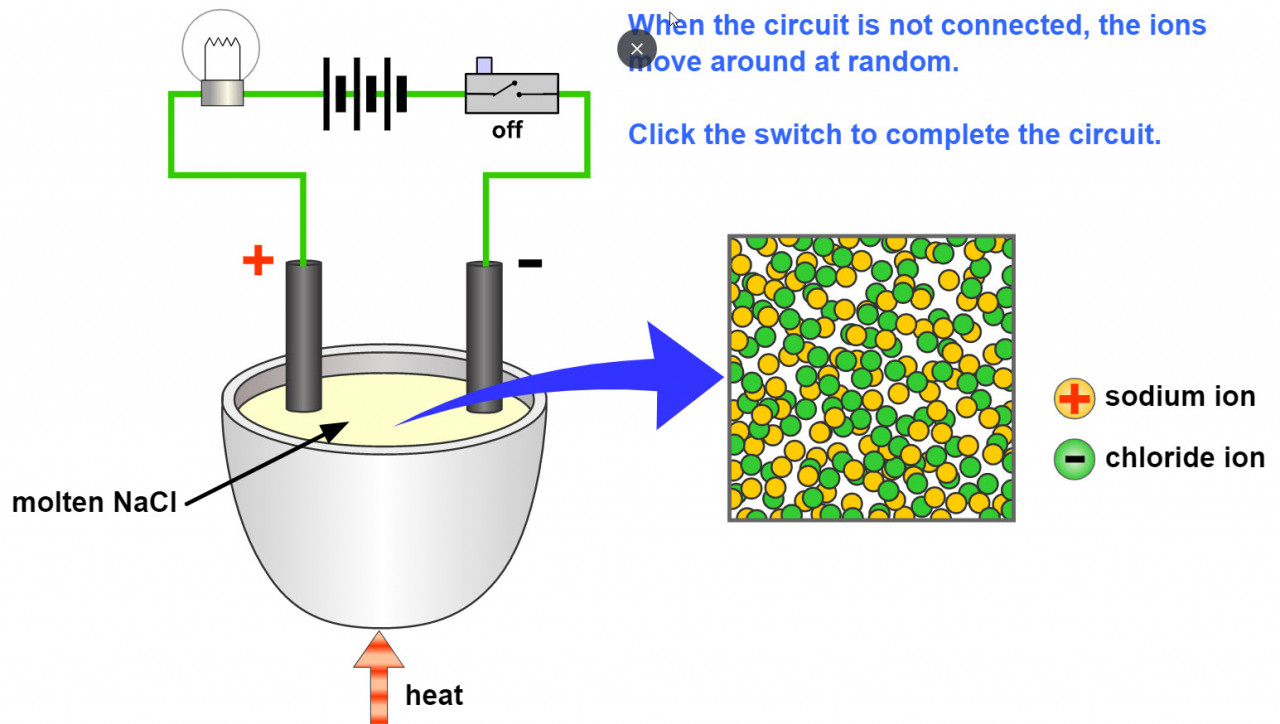
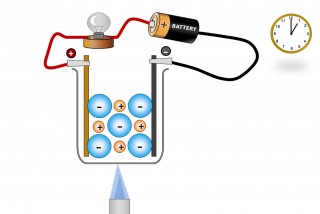
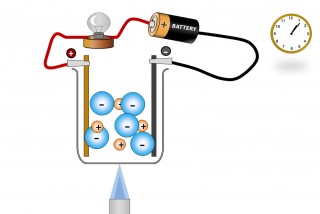
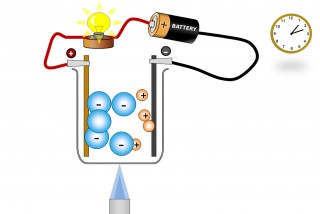
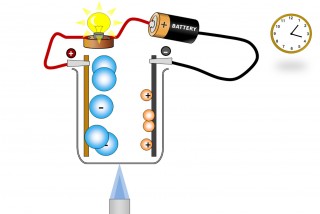
A liquid substance containing ions which are mobile can conduct an electric current. When this happens chemical changes take place at the electrodes. Electrons are given off at the negative electrode ( cathode) and electrons are taken away from the positive electrode ( anode).
The ions ( which are mobile because they are molten or dissolved) will migrate as the electric field is applied. The positive ions (Anions) will migrate to the negative electrode and the negative ions (Cations) migrate to the positive electrode.
chemical changes then take place at the electrodes and a current flows.
Covalently bonded compounds do not contain ions so that even when molten ( so that the molecules are free to move) , there is no migration of particles and no conduction.
1.58 Activity 2. Electrolysis of molten ionic compounds
Students should:
- 1.58C describe experiments to investigate electrolysis, using inert electrodes, of molten compounds including lead(II) bromide and aqueous solutions including sodium chloride, dilute sulfuric acid and copper(II) sulfate and to predict the products
1.59 Activity 3. Half equations
Students should:
- What is the half equation for the reduction of sodium ions at the cathode?
- Why is it called "reduction" ?
- What is the product?
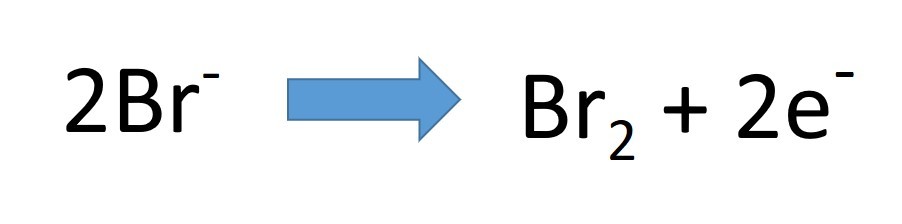
- What is the half equation for the oxidation of chloride ions at the anode?
- Why is it called "oxidation" ?
- What is the product?
Enter your text here ...
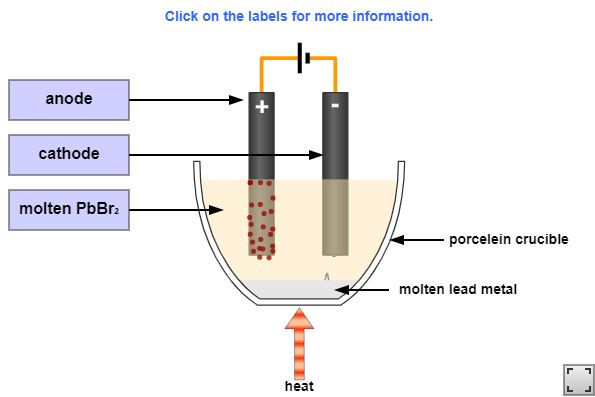
1.58 Activity 4. A toxic separation
The electrolysis of molten lead bromide is a popular electrolysis reaction and is often shown in schools. However it is dangerous and needs to be performed in controlled conditions. Watch the this video carefully to find out why.
- What ions are present in lead bromide?
- Which two elements is lead bromide broken down into?
- What element appears as an orange vapour?
- Why does it appear as a vapour rather than a liquid?
- Why is this process dangerous?
Try to write half equations for the reaction at each electrode.
This video might help you https://youtu.be/iKwEnGPboNc
- The ions present are lead and bromide. Pb2+ and Br-.
- Lead Bromide is broken down into its elements; lead and bromine.
- Bromine appears as orange vapour.
- The Bromine produced is so hot, gas is immediately formed.
- Bromine gas is an unpleasant, corrosive substance and lead is toxic.

Electrolysis of copper sulfate solution using copper electrodes
The Lead Tree
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.