1.19 Electron configurations
1.19 Deducing electron configurations
Students should:
1.19 understand how to deduce the electronic configurations of the first 20 elements from their positions in the Periodic Table
A carbon atom has 6 protons and therefore 6 electrons. The electrons are arranged in two shells; 2 electrons in the first shell and 4 electrons in the second shell. The electron configuration of a carbon atom can be therefore represented as: 2, 4
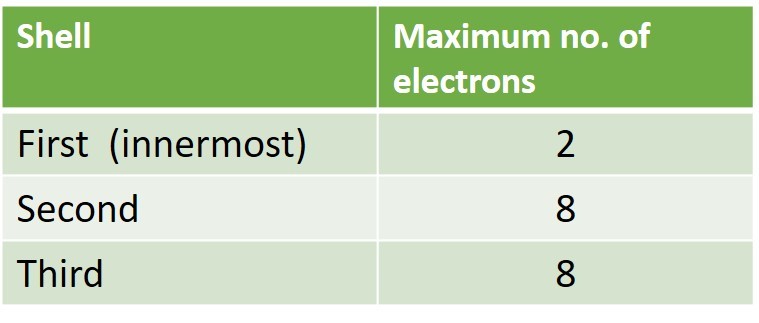
The electrons in an atom arrange themselves in shells ( or levels) around the nucleus. The electrons fill the innermost shells first . Each shell can contain a maximum number of electrons.
This model works well for explaining the properties of the first 20 elements in the periodic table - a more complex model is needed to explain the structure of atoms above atomic number 20.
1.19 Activity 1. Looking for patterns
Filling up nicely.
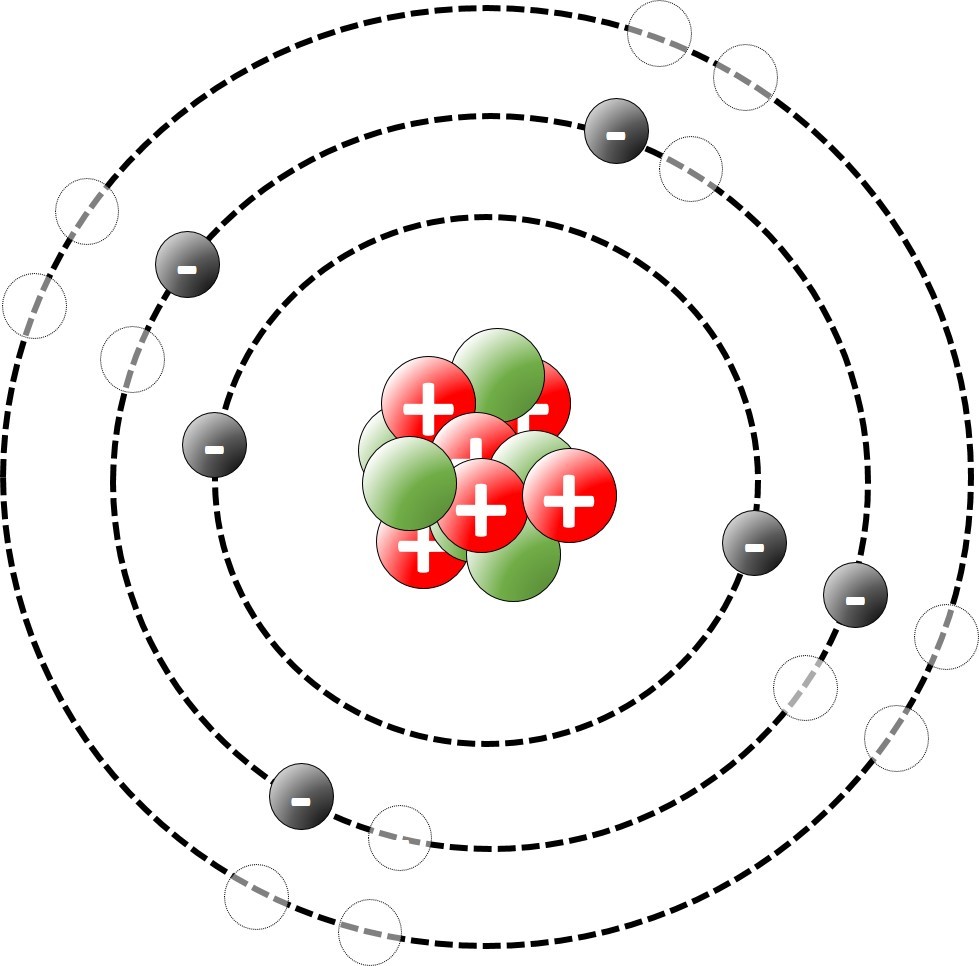
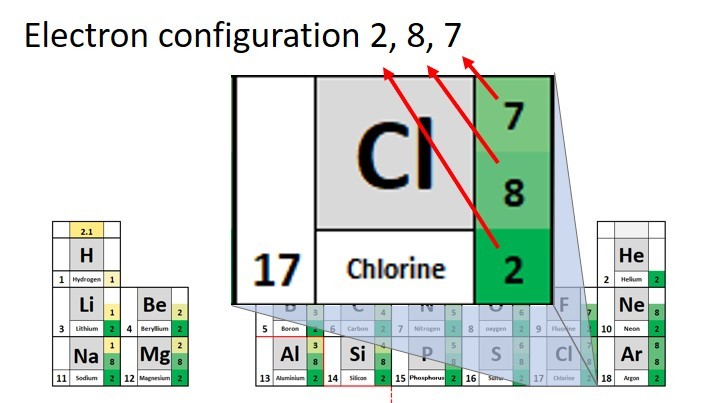
Chlorine has an atomic number of 17. This means that the atoms have 17 protons. Because atoms have no overall charge the number of electrons must also be 17. These are arranged as follows:
Starting with the innermost shell .
2 electrons ( maximum for shell 1)
8 electrons ( maximum for shell 2)
7 electrons left over = (17 - (8 + 2))
Question.
The atomic number of Potassium is 19 and that of Calcium is 20. What is the electron configuration for each atom?
Answers: Potassium K, Calcium Ca
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.