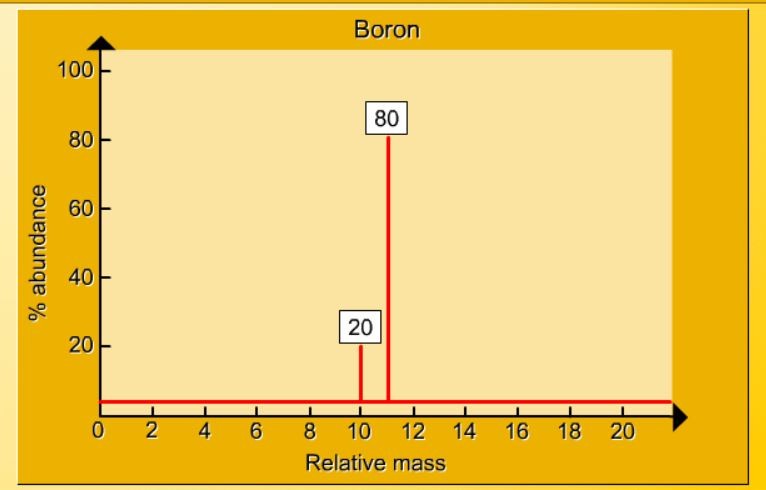
1.17 Activity 2. Calculating relative atomic mass. Students should: 1.17 be able to calculate the relative atomic mass of an element (Ar) from isotopicabundances. Sorting by mass A mass spectrometer is an instrument which can separate and sort the particles in a sample of an element according to their mass. The output from a ...
1.16 Activity 1. Three types of carbon atom Students should: 1.16 know what is meant by the terms atomic number, mass number, isotopes and relative atomic mass (Ar). Use this animation to explore the number and type of sub atomic particles which make up the three isotopes of carbon. Use the information to complete a copy of th...
If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
Ionic compounds form when the atoms of a metal combine with the atoms of a non - metal
Atomic models Atoms are the building blocks of matter. Atoms are the smallest possible particles into which an element can be subdivided, without changing its properties. As building blocks, they can be thought of spheres. However, to understand how and why atoms join up with one another, a more sophisticated model is needed....
Sun and air? Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAirGAS and PLASMASun But as a chemist you might be as...
Concentration Noun :the action or power of focusing all one's attentiona close gathering of people or things.the relative amount of a particular substance contained within a solution or mixture or in a particular volume of space In this topic we will of course be using the third definition of concentration. Students should: 1.34C ...
1.34 understand that ionic compounds have high melting and boiling points because of strong electrostatic forces between oppositely charged ions1.35 understand the relationship between ionic charge and the melting point and boiling point of an ionic compound1.36 describe an ionic crystal as a giant three-dimensional lattice structure held together...
Crystallisation can be used to separate a solid solute from its solvent. Re-crystallisation is used to purify samples obtained in the laboratory. But what is a crystal?
In this section we consider how much product is formed by a chemical reaction . Some reaction processes are not very efficient and only a small proportion of the reactants are actually turned into the products. The yield of a chemical reaction is the amount of product obtained. It is usually expressed as a perce...